General description
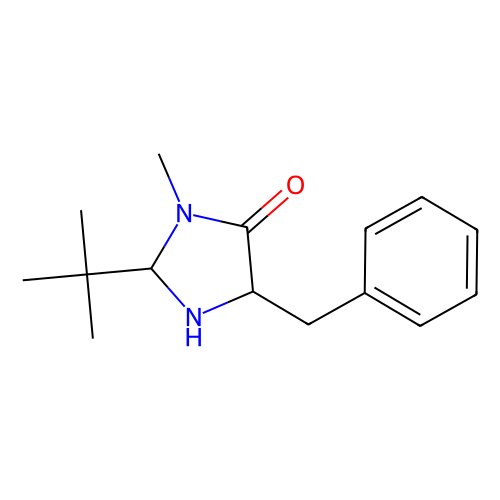
(2S,5S)-(-)-2-tert-Butyl-3-methyl-5-benzyl-4-imidazolidinone is a chiral imidazolidinone organocatalyst, developed by MacMillan and co-workers.
Application
(2S,5S)-(-)-2-tert-Butyl-3-methyl-5-benzyl-4-imidazolidinone is a second-generation MacMillan catalyst, which can be used as a chiral organocatalyst in:
- The chiral transformation reaction, including Friedel-Crafts and Mukaiyama-Michael reactions.
- The preparation of substituted spiroundecenetriones via asymmetric domino Knoevenagel/Diels-Alder reactions.
- The asymmetric synthesis of β-hydroxy aldehydes and their dimethylacetals via aldehyde-aldehyde aldol condensation reaction.
- The enantioselective α-fluorination of aldehydes using N-fluorobenzenesulfonamide as a fluorinating agent.
- The stereoselective preparation of (oxomethyl)oxabicyclo[3.2.1]octenones and tricyclic pyrroles via [4+3] cycloaddition of (trialkylsiloxy)pentadienals to furans.
Metal-free OrganoCatalyst technology for asymmetric catalysis. Catalyzes asymmetric indole alkylations, Friedel-Crafts alkylations, and a broad range of conjugate addition reactions in high enantiomeric excess.
Features and Benefits
Advantages of MacMillan imidazolidinone organocatalysts:
- Superior enantiocontrol in numerous transformations
- High activities at low catalyst loadings
- Extraordinary functional group tolerance
Legal Information
U.S. Pat. 6,369,243 and related patents apply. For research purposes only.
- UPC:
- 51451700
- Condition:
- New
- Availability:
- 3-5 Days
- Weight:
- 1.00 Ounces
- HazmatClass:
- No
- MPN:
- 663107-500MG
- CAS:
- 346440-54-8